Your guide to global food and feed safety
/Asiantuntijuus/food-safety-consulting-5b-biosafe-1000x1000.jpg?width=1920&name=food-safety-consulting-5b-biosafe-1000x1000.jpg)
/Lopputuote/microbial-products-8-biosafe-1000x1000.jpg?width=512&name=microbial-products-8-biosafe-1000x1000.jpg)
Microscopic focus. Macroscopic safety.
Leading and consulting the safety assessment of microbial food and feed products.
-
We help our customers from product development to dossier submission to enable and authorization process to enable efficient, quick and smooth market access.
-
Focus on biotechnological products and microbes used as such or as production organisms.
Competence
-
Deep knowledge of EU, US and global legislation as well as EFSA and FSA guidance requirements
-
Long experience in laboratory in vitro- and bioinformatic analyses
-
Services designed to comply with the requirements of the food safety authorities
Business areas
Food & Feed Additives
Food enzymes
Novel foods and food microbes
Plant protection products
+ microbes used in other applications
Decades of experience in food safety assessment, laboratory research, and legislation
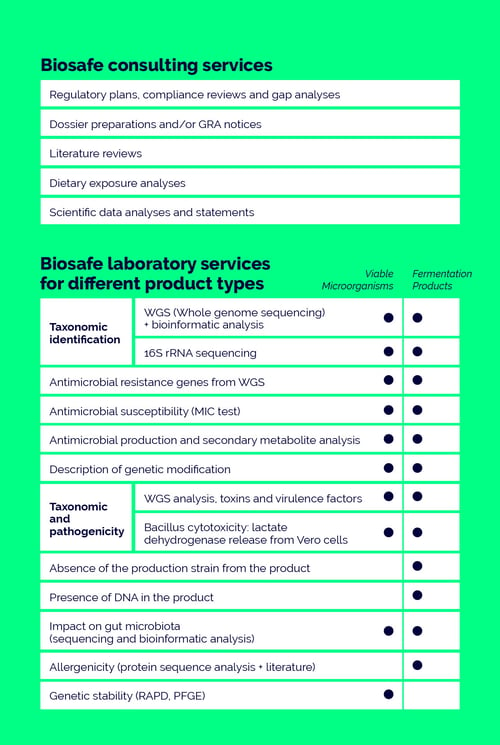
We are a one-stop-shop for safety contract research organisation (CRO) specialising in safety assessments of biotechnological and microbial foods, food and feed additives, food enzymes, probiotics or biopesticides and professional consulting on successful market entry into the EU, US and elsewhere.
EU regulations require new products and ingredients require safety assessment before they can enter the EU, US or other markets. We can support you at all steps in the process, whether it's early-stage laboratory research or seeking approval for an already developed product.
-
Get your products to market faster
-
Simplify your process and cut costs
-
Manage risks with professional guidance
Your path to approved studies — what happens after you contact us?
What you do:
1.
Contact us
To clarify your needs.
What we do:
2.
Personalised kick-off meeting and a quotation
Prepared by our experts to fulfil the requirements of EFSA for your product. Annual offer possible.
What you do:
3.
Approve the quote and send your samples
We'll send instructions on how to prepare and ship your samples.
What we do:
4.
We'll test your sample in our laboratory and analyse the data
We will tell you how the study is progressing or of unexpected results.
We can consult with EFSA too and notify them about the study.
What you do:
5.
Get your study report and review the results with us
We will go over the first draft report with you and amend it based on your comments. We can help with any questions from EFSA. You'll get a self-explanatory, EFSA-compliant full study report to be used as such in your dossier.
What we do:
6.
We’ll dispose of your samples
Biosafe ensures the sample is disposed of according to regulations or we can store them longer if needed.
Receive this
all-inclusive guide in your email
Tell us your situation and
let's get going.

Pauliina Halimaa
Managing Director, PhD
+358 40 640 3225
pauliina.halimaa@biosafe.fi

